Officials eager for FDA approval on antibody tests to combat virus
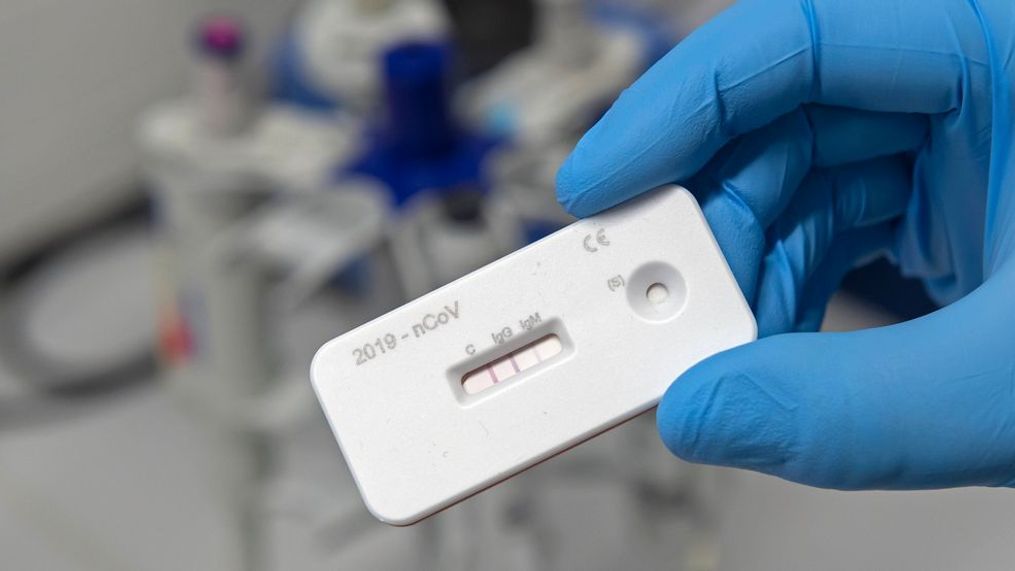
WASHINGTON (SBG) — Vice President Mike Pence is optimistic an antibody test will be approved by the Food and Drug Administration "in just a matter of days" to combat the ongoing coronavirus pandemic.
In addition to case surveillance and testing for active cases, antibody testing is considered a pillar in a safe reopening of the country.
“In order to really be able to gain as much information about the pandemic, we need to know who has it, who’s recovered, where it is, being able to trace it," Dr. Azadeh Shirazi, a surgeon in California said.
The test will determine if a person had the virus and recovered. In addition to knowing they have at least short-term immunity, a person who's recovered from COVID-19 can help save lives by donating their plasma.
In the state of Washington, Elizabeth Schneider is one of the first to donate her plasma to help someone suffering from the coronavirus.
“We have an immune system that was able to fight this off on our own and that’s really, really powerful," Schneider said.
Some are skeptical about how close the United States is to achieving a system of widespread antibody testing.
Dozens of developers are working on creating an antibody test, which are mainly done with a finger-prick, but none of them have been approved by the FDA. One has been given emergency use authorization.
At the White House, there's been mixed messaging about when the tests will be available. Assistant Secretary for Health Admiral Brett Giroir said last week the tests should be ready by May. Just days later, Dr. Anthony Fauci said they would be ready within a week.
Giroir said during a briefing last week that he's worried about how accurate the tests will be.
“It is very important that they do what they say they do and we have reason to believe that not all of them are going to perform well," Giroir said.“We’re going to be very careful to make sure that when we tell you you’re likely immune from the disease, you’re really, that test really said that.”
The Trump administration announced over the weekend it will require health insurers to cover the cost of antibody testing.
